abbott point of care covid test
Abbotts rapid tests are among the most widely-used in the US with more than 200 million of our BinaxNOW and ID NOW rapid tests used in urgent care clinics doctors offices pharmacies nursing homes and schools since April 2020. Our Rapid COVID-19 Tests Our BinaxNOW test is the size of a credit card and requires no specialized instrumentation.
Abbott S Point Of Care Covid 19 Test Detects Coronavirus In As Little As 5 Minutes Biospace
Results from the simple nasal swab are available in 15 minutes through testing individuals suspected of COVID-19.

. The availability and ease-of-access of ID NOW which delivers results in minutes rather than a day or more is helping to reduce the spread and risk of infection by. Bộ xét nghiệm không cần kê đơn này rất. This document provides a step-by-guide to get started with Point of Care POC COVID-19 antigen testing.
Thus despite the higher sensitivity and specificity of the RT-PCR assays the impact of POC tests cannot be ignored. Our ID NOW test for COVID-19 is the fastest molecular point-of-care rapid test available today and has been delivering reliable results when and where theyre needed. To help provide the critical diagnostic information needed Abbott is currently providing and.
T his test is authorized for use at the Point of Care POC ie in patient care settings operating under a CLIA Certificate of Waiver Certificate of Compliance or Certificate of Accreditation. To capture these results the Indiana Department of Health IDOH has developed the COVID-19 Point-of-Care Test Reporting - Indiana Department of Health REDCap form. Ensure the test is administered in qualified point-of-care setting by trained personnel The EUA for the Abbott BinaxNOWCOVID-19 Ag card test allows for use in point-of-care settings that are qualified to have the test performed and are operating under a CLIA Clinical Laboratory Improvement Amendments Certificate of Waiver Certificate of Compliance or.
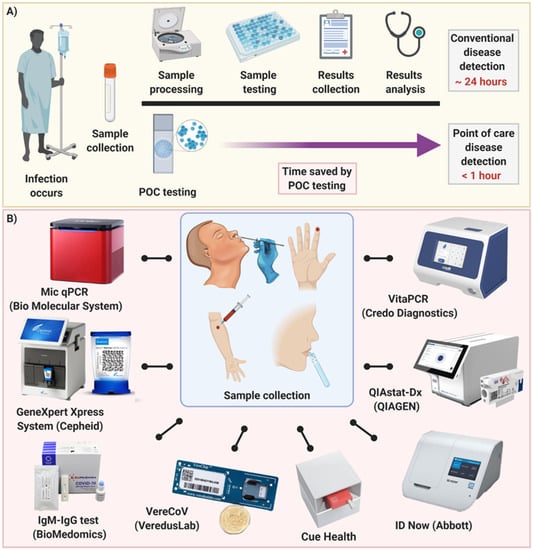
Point-of-care testing for SARS-CoV-2 has become an increasing used testing methodology for diagnosing patients with COVID- 19 in a variety of settings. Ad We Carry A Wide Range Of Testing Kits Instruments Supplies And More. The BinaxNOW COVID-19 Antigen Self Test is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 from individuals with or without symptoms or other epidemiological reasons to suspect COVID-19 infection when tested twice over three days with at least 36 hours between tests.
Abbott has received emergency use authorization EUA from the US. The tests can be used in point-of-care settings and at home with an online service provided by eMed. Store between 356-86 F 2-30 C until use.
The BinaxNOW COVID-19 Antigen Self Test is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 from individuals with or without symptoms or other epidemiological reasons to suspect COVID-19 infection when tested twice over three days with at least 36 hours between tests. ID NOW is an FDA approved CLIA-waived instrument which means that. Our COVID-19 unit is now making Abbott BinaxNOW COVID-19 antigen card point of care POC test kits BinaxNOW test kits available for use at nursing facilities and assisted living residences both referred to hereafter as long-term care.
Our Alcohol And Drug Testing Products Are The Most Precise Easy-To-Use On The Market. The COVID-19 pandemic is affecting all of us around the world. 2 BinaxNOW COVID-19 Antigen Test Cards 2 Nasal Swabs 2 Reagent Bottles.
This form will allow all settings. As a leader in diagnostic testing we have a unique responsibility to contribute our expertise to help fight the COVID-19 pandemic. The ID NOW COVID-19 test is a rapid molecular point-of-care test that detects COVID-19 in 13 minutes or less.
Testing at the point-of-care POC for COVID-19 adds a distinct advantagerapid availability of results upon which to make treatment and infection prevention and control decisions. A rapid test for the qualitative detection of COVID-19 antigens in nasal swab specimens. However faster antigen tests and other point-of-care POC devices have also played a significant role in containing the spread of SARS-CoV-2 by facilitating mass screening and delivering results in less time.
The clinical performance of POC tests depend on the circumstances in which they are used and how carefully the test is performed. It is used on our ID NOW platform. Diagnostics Testing May 27 2020.
Abbott received emergency use authorization EUA from the US. 9125 L x 0938 D x 5063 H. Depending on the test manufacturers instructions for use which can be found on FDAs EUA website external icon.
A CLIA-certified laboratory or testing site must report all SARS-CoV-2 diagnostic and screening test results to the person who was tested or that persons healthcare provider. Các xét nghiệm Panbio COVID-19 được các chuyên gia chăm sóc sức khỏe ở hơn 100 quốc gia sử dụng. Abbotts rapid COVID-19 test isnt the only point-of-care test to receive FDA authorization during the pandemic but Trump has touted it the most by far hailing the speed at which results can.
Kit contains all necessary components for testing including. The Abbott PanBio TM COVID-19 Ag point-of-care test was performed alongside RT-PCR. Currently the Virginia Department of Health VDH offers one type of prescription antigen test.
Nhanh chóng đơn giản và đáng tin cậy Panbio COVID-19 Antigen Self-Test cung cấp kết quả sau 15 phút tại nhà. This study recruited participants presenting for COVID-19 testing at three Melbourne metropolitan hospitals during a period of low COVID-19 prevalence. Abbotts BinaxNOW COVID-19 Ag Card test can identify these antigens which are typically detected after symptoms start.
PANBIO COVID-19 ANTIGEN SELF-TEST. In addition participants with COVID-19 notified to the Victorian Government were invited to provide additional swabs to. Abbott BinaxNOW COVID-19 Antigen Ag Card A self-contained antigen test that uses a card and does not require a separate analyzer device.
Food and Drug Administration FDA for the ID NOW COVID-19 test in March 2020. Abbott is putting its resources towards helping you navigate this crisis. Food and Drug Administration FDA for the fastest available molecular point-of-care test for the detection of novel coronavirus COVID-19 delivering positive results in as little as five minutes and negative results in 13 minutes.
What makes this test so different is where it can be used. Reporting Requirements for Rapid Testing in Point-Of-Care Settings. According to Abbott the rapid test which runs on the ID NOW platform is an.
The Food and Drug Administration FDA has issued an Emergency Use Authorization for the Abbott ID Now COVID-19 test a molecular point-of-care test that delivers results within minutes allowing healthcare professionals to make clinical decisions during a patient visit. Term care residents for COVID-19.

Demand For Abbott Labs Covid 19 Tests Soars Past 40 Million As Pandemic Cases Surge
Diagnostics Free Full Text Point Of Care Diagnostics In The Age Of Covid 19 Html

Steps To Use Id Now Effectively Abbott Newsroom

Abbott Labs Rapid 5 Covid 19 Test To Fill In Testing Gaps For Millions In The U S

Id Now Covid 19 Abbott Point Of Care

Our Quick Guide To Rapid Covid 19 Testing Abbott Newsroom

Point Of Care Testing Diagnostics Testing Newsroom
Virus News Abbott Launches 5 Minute Covid 19 Test Bloomberg
Image Gallery Showing Impact Of The Covid 19 Pandemic Daic

Abbott Labs Has Shipped 566 000 Rapid Covid 19 Tests To All 50 U S States

Abbott Id Now Covid 19 Detection Test System Us

As Problems Grow With Abbott S Fast Covid Test Fda Standards Are Under Fire Kaiser Health News
How Rapid Tests Are Being Used To Test For Covid 19 Across Canada Globalnews Ca

Abbott On Twitter We Re Launching A Molecular Point Of Care Test That Delivers Positive Covid 19 Results In As Little As 5 Minutes And Negative Results In 13 Minutes Providing Information Where It Is Needed

Abbott Id Now 2019 Ncov Testing

14 000 Rapid Covid 19 Testing Kits Coming To Grey Bruce Ctv News

Fda Authorizes Covid 19 Test That Doesn T Need Special Equipment Los Angeles Times

Nyu Study Flags False Negatives From Abbott S Portable Coronavirus Test While Fda Lists Concerns Fierce Biotech
